Since 2007, when it received Japan's first marketing approval for a regenerative medical product, Japan Tissue Engineering Co., Ltd. (J-TEC) has provided a stable supply of high-quality products with guaranteed efficacy and safety.
Under the motto of making regenerative medicine a standard healthcare, J-TEC engages in manufacturing and quality assurance activities that ensure product safety and promote the evolution of regenerative medicine from a niche market to a familiar form of treatment.
Construction of a production system for realizing a stable supply of high-quality products
Autologous regenerative medical products are made-to-order products produced from the tissue of individual patients. Because such products cannot be made over, stable manufacture of high-quality products is imperative. J-TEC also supplies products to support R&D on topical pharmaceuticals and cosmetics. All of these products are stably manufactured and tested according to prespecified procedures by workers who have received extensive education and training.

J-TEC Head Plant (right) and Research Facility (left)
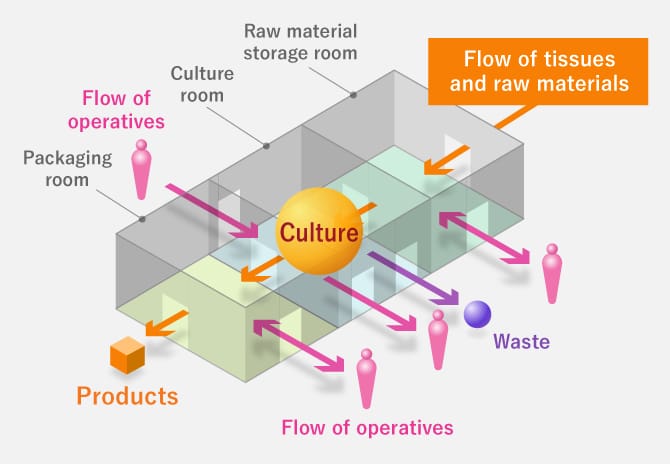
Image Layout of Facilities
Efforts to make a stable supply of products possible
Manufacture

The manufacturing work is performed by workers who have acquired specialized knowledge and skills in cell culturing through education and training. We strive to maintain and improve workers' knowledge and skills by encouraging them to obtain the qualification of JSRM Cell Processing Operator and establishing our own in-house Meister system.
Quality control

Quality testing based on the latest findings is performed at every stage from acceptance of raw materials to release of products to the market. We strive to maintain and improve workers' knowledge and skills by encouraging them to obtain the qualification of JSA's QC KENTEI (Quality Management and Quality Control Examination) and establishing our own in-house Meister system.
Production control

J-TEC strives to deliver products reliably by coordinating delivery schedules to reflect the characteristics of products that use a patient's own cells.
Purchasing control

J-TEC has constructed a thorough control system for the procurement, acceptance, and storage of raw materials and packaging and labeling materials, ensuring traceability through collection of information from suppliers and in-house code management.
Production technology
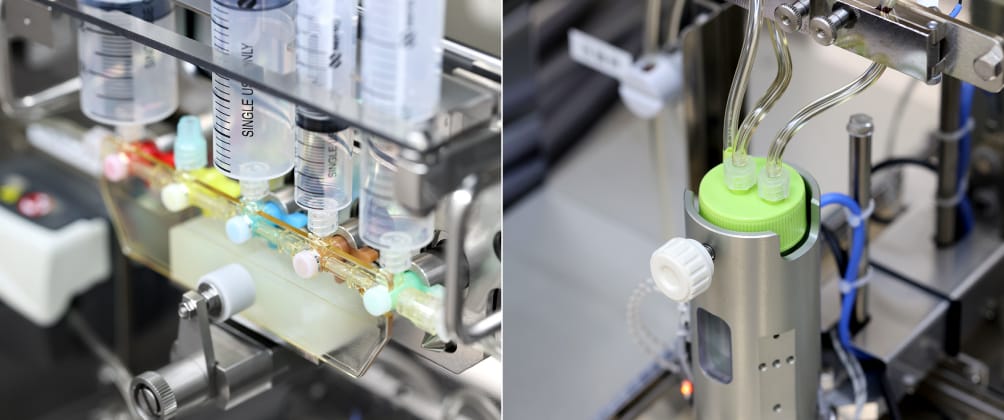
J-TEC provides technical support to efficiently and stably realize production of multiple autologous cultured products. For example, we have developed compact automated and mechanical equipment and operator support tools for high-burden processes that operators with advanced skills must engage in for long hours.
Maintaining a clean environment to ensure safety and security
Safety cabinets in clean rooms*1 that can maintain an aseptic operating environment*2 through the use of special filters*3 have been introduced to ensure clean handling of the patient's tissue and cells.
- Class 10,000: A clean environment, with less than 10,000 particles of diameter 0.5 micrometers or greater per cubic foot of air.
- Class 100: An extremely clean environment, with less than 100 particles of diameter 0.5 micrometers or greater per cubic foot of air.
- HEPA filter: A High Efficiency Particulate Air (HEPA) filter purifies the air by removing particles of diameter 0.3 micrometers or greater with an efficiency of 99.97% or more. It is necessary for clean environment and sterile operations.

Aseptic processing in safety cabinet

Clean Room Corridor
Strict facility supervision and monitoring for improved product quality
The operating status of the air conditioning equipment for maintaining the clean environment and the cell culturing equipment, etc. is monitored 24 hours a day, and records of all operations are retained. A system has been constructed whereby an alarm will sound and the manager will be notified in the unlikely event of an equipment anomaly. Moreover, a security system has been introduced to restrict entry to manufacturing facilities and make it possible to check who has entered and the status of work via webcam, which has been useful in improving the quality of the products.

Monitoring facilities

Monitoring air conditioning equipment
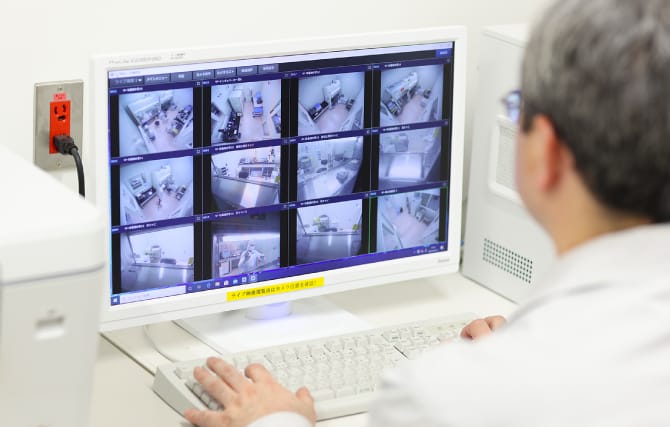
Checking entry and status of work via webcam
Constructing a quality assurance system that guarantees safety and security
In order to achieve customer satisfaction, we must provide safe, secure, high-quality regenerative medical products and regenerative medicine related services. To satisfy these requirements, we have established a "Quality Policy", and we are constructing a quality assurance system to enable us to expand our business responsibly as the leading company in the regenerative medicine industry.
Quality Policy
As the leading company in regenerative medicine, Japan Tissue Engineering Co., Ltd. has established the Corporate Philosophy of "contributing to an improved quality of life for patients, while complying with legal statutes and ethical guidelines", and we do business on the basis of this policy in order to provide high-quality regenerative medical products and services.
- 1J-TEC aims to satisfy all patients and healthcare professionals and win their trust as a corporation that fulfills a need in society.
- 2J-TEC pursues the highest global standard of quality in regenerative medicine through technological innovation.
- 3J-TEC is a highly ethical corporation that complies with regenerative medicine related laws, regulations, and rules.
- 4J-TEC properly provides accurate information to all patients and healthcare professionals.
- 5J-TEC values the voice of people at the front lines of regenerative medicine, and we are constantly striving to improve our quality.
J-TEC performs R&D and clinical trials, manufacture and testing, and marketing and post-marketing management in accordance with the ordinances and notifications issued by the Ministry of Health, Labour and Welfare. A Quality Assurance Department independent of these other departments has centralized control over operations related to quality assurance for studies conducted in the course of R&D, review of clinical trials, post-marketing quality assurance, safety management, and post-marketing surveillance, and handles the quality assurance activities involved.
Quality Management System
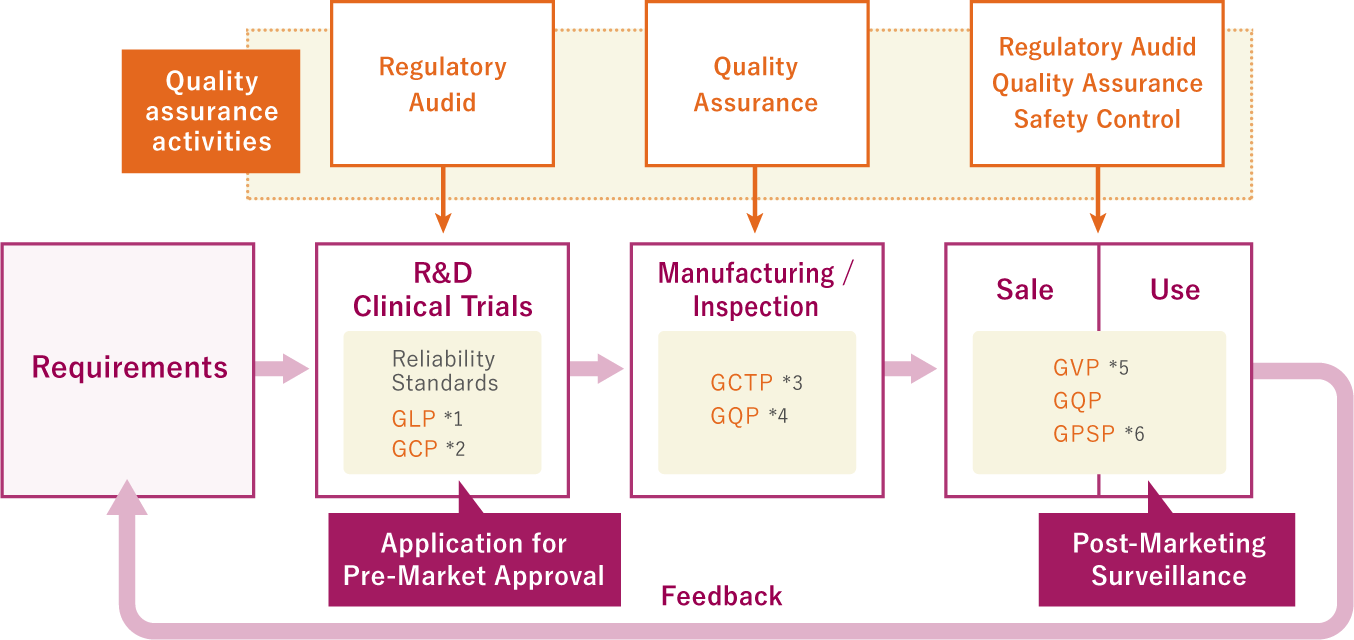
- GLP: Ordinance on standards for non-clinical trials related to the safety of tissue-engineered medical products
- GCP: Ordinance on standards for clinical trials of tissue-engineered medical products
- GCTP: Ordinance on standards for quality and manufacturing control of tissue-engineered medical products
- GQP: Ordinance on quality assurance standards for pharmaceuticals, quasi-drugs, cosmetics,and tissue-engineered medical products
- GVP: Ordinance on post-marketing safety control standards for pharmaceuticals, quasi-drugs, cosmetics, medical devices, and tissue-engineered medical products
- GPSP: Ordinance on standards for post-marketing surveillance and testing of tissue-engineered medical products
Quality assurance activities
Pharmacovigilance activities
The approval of the Ministry of Health, Labour and Welfare is required for the manufacture and marketing of regenerative medical products. An enormous amount of documentation is submitted in the marketing approval application dossier and reviewed. The handling of the study data that form the basis of that documentation is extremely important. Moreover, the reliability of the post-marketing surveys and studies must also be ensured.
At J-TEC, the department for regulartory audit surveys and reviews non-clinical studies and clinical trials to ensure the reliability of studies on the efficacy and safety of the products. It also checks whether post-marketing quality assurance activities, safety management activities, and surveillance activities are being conducted properly and ensures their reliability.

Quality assurance activities
In order to maintain product quality, it is necessary to perform manufacture and testing according to prespecified procedures. The maker must also collect and analyze information on product quality from medical institutions, etc. and take appropriate action as needed.
At J-TEC, the Quality Assurance Department is striving to ensure the quality of products by monitoring whether manufacturing control and quality control activities are being conducted properly, confirming that manufacturing quality is maintained, analyzing and investigating quality information collected from medical institutions, etc. in collaboration with related departments, and making any necessary improvements.

Safety management activities
In order to ensure the safety of regenerative medical products, it is necessary to collect and analyze information on product safety from medical institutions, etc. and take appropriate safety assurance measures as needed.
At J-TEC, the department for safety controlis striving to ensure the safety of patients by analyzing and investigating information collected from medical institutions, etc. and taking any necessary action rapidly and reliably, such as reporting the information to the regulatory authorities or providing additional information to medical institutions.

Post-marketing surveillance activities
As a general rule, makers of regenerative medical products are required to conduct post-marketing surveillance activities for a set period of time following approval, during which data is collected on the patients who have used the products at medical institutions to reconfirm the approved indication, efficacy, and safety.
At J-TEC, the department for safety control analyzes the patient data collected from medical institutions, confirms indication, efficacy, and safety annually, and reports the results to the regulatory authorities. After the set period of time has ended, the results of this confirmation are summarized, and an application for reexamination is made.

