Autologous Cultured Oral Mucosal Epithelium
Autologous Cultured Oral Mucosal Epithelium is a product for the treatment of limbal stem cell deficiency, and it is the world’s first regenerative medical product using oral mucosal epithelial cells to treat this disease. This product will be manufactured in Japan and was developed through the practical application of technology developed by Prof. Kohji Nishida of the Department of Ophthalmology, Osaka University Graduate School of Medicine. It will be Japan’s second regenerative medical product in the ophthalmology field, following Autologous Cultured Corneal Epithelium “Nepic”, for which marketing approval was obtained in March 2020.
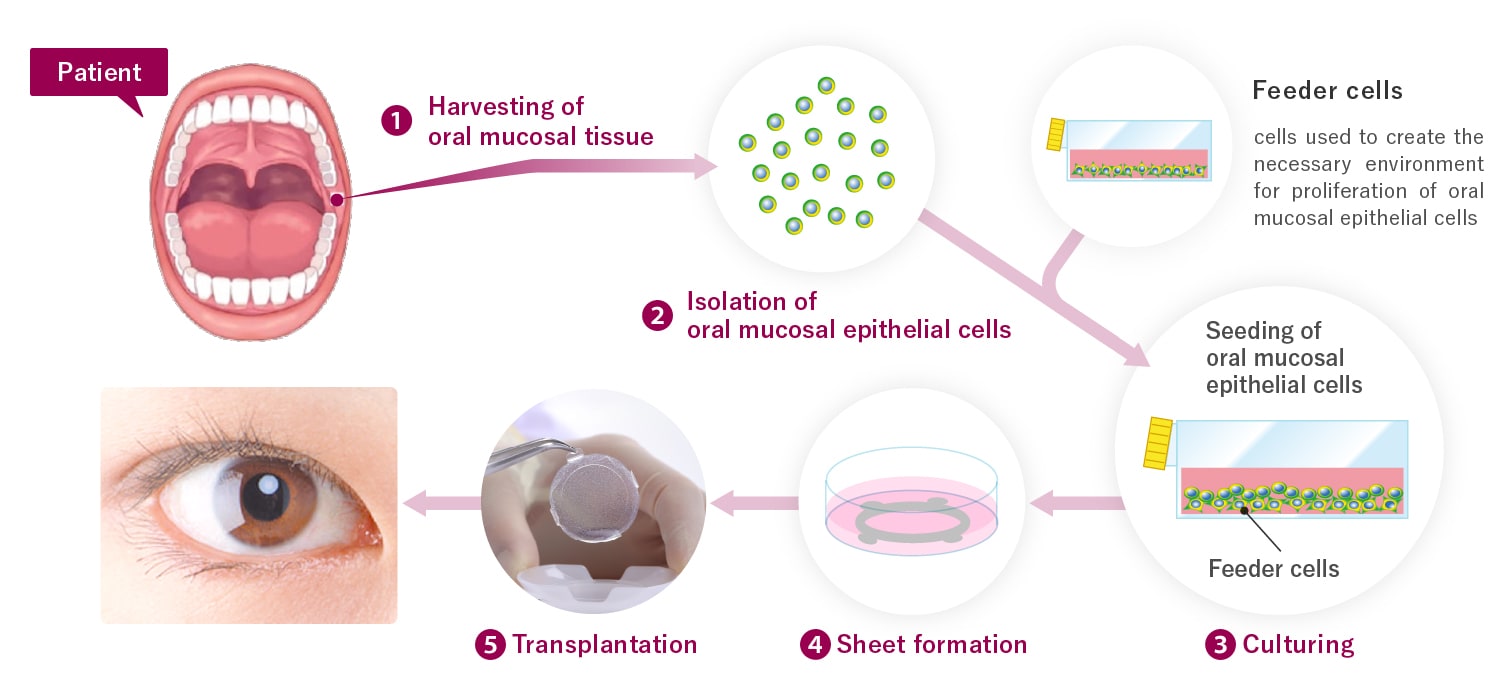
Autologous Cultured Oral Mucosal Epithelium, the product for which marketing approval was obtained this time, is a sheet of epithelial cells derived from human (autologous) oral mucosa that is manufactured by harvesting the patient’s own oral mucosal tissue and culturing the cells isolated from it. The purpose of the product is to repair damaged corneal epithelium, and when this product is transplanted onto the surface of the patient’s eyes, the patient’s own oral mucosal epithelial cells become engrafted and epithelialize. Autologous Cultured Oral Mucosal Epithelium is a promising new treatment method for patients who have extensive damage to the cornea of both eyes from limbal stem cell deficiency and have markedly reduced visual acuity. Autologous Cultured Oral Mucosal Epithelium was designated a regenerative medical product for the treatment of rare diseases in 2020, with the indication of limbal stem cell deficiency.

Cultured Oral Mucosal Epithelium
Development
In the development of this product, an investigator-initiated clinical trial was conducted by Prof. Kohji Nishida and Lecturer Yoshinori Oie et al of Osaka University Graduate School of Medicine (Department of Ophthalmology) with support from AMED.1) J-TEC introduced the autologous cultured oral mucosal epithelial cell sheet transplantation technology that Dr. Kohji Nishida led the world in developing, and took over the investigator-initiated clinical trial, conducting it as a corporate sponsored clinical trial since September of 2016. We applied for marketing approval in September of 2020, and marketing approval was obtained for Autologous Cultured Oral Mucosal Epithelium in June, 2021.
Limbal stem cell deficiency treatments using Autologous Cultured Oral Mucosal Epithelium have been covered by insurance since December 1, 2021.
1) Japan Agency for Medical Research and Development
Culture
Autologous Cultured Oral Mucosal Epithelium is manufactured by harvesting the patient’s own oral mucosal tissue and culturing the cells isolated from it.
Transplantation of autologous cultured oral mucosal epithelium
The use and commercialization of the medicinal products developed by Japan Tissue Engineering Co., Ltd. that are referred to on this website are approved only in Japan. A potential use and commercialization in other regions will be subject to the prior granting of a marketing authorization in the given territory and compliance with applicable laws.
Researchers
- Kohji Nishida, M.D., Ph.D., Professor, Department of Neural and Sensory Organ Surgery (Ophthalmology), Osaka University Graduate School of Medicine Osaka University Graduate School of Medicine


