Jan. 29, 2025[PICK UP]
Results of Joint Research on In Vitro Skin Irritation Tests Received the President's Special Award
Results Presented at the 37th Annual Meeting of the Japanese Society for Alternatives to Animal Experiments
Japan Tissue Engineering Co., Ltd. (J-TEC, headquarters in Gamagori, Aichi Prefecture; President and CEO: Ken-ichiro Hata) manufactures and markets cultured human tissue for research use (human 3-dimensional cultured tissue) that is utilized for in vitro skin irritation tests and other purposes. J-TEC, along with a total of five other companies – Kobayashi Pharmaceutical Co., Ltd., Sunstar Group, TOA Corporation, Mandom Corporation, and ROHTO Pharmaceutical Co., Ltd. – presented results of joint research on alternatives to the in vitro skin irritation test, one of the safety evaluation items, at the 37th annual meeting of the Japanese Society for Alternatives to Animal Experiments. The presented results received the President's Special Award.
According to the guidance that the Ministry of Health, Labour and Welfare issued in April 2021 regarding skin irritation evaluation systems for quasi-drugs and cosmetics, the ingredients of quasi-drugs and cosmetics that may be used for tests are limited only to those that have almost no potential risk of skin irritation. We have been working to provide more data on alternatives to animal experiments and improve test conditions for the purpose of revising the guidance so as to expand the scope of the ingredients that can be used for tests. Results of this research were presented and received the President's Special Award at the 37th annual meeting of the Japanese Society for Alternatives to Animal Experiments held at Light Cube Utsunomiya from November 29 (Friday) to December 1 (Sunday) 2024.
In obtaining results of this research, we received the Japanese Society for Alternatives to Animal Experiments's 8th research grant concerning test method evaluation aimed to ensure the safety of cosmetics and other products. KOSÉ Corporation, SSCI-Net, and Japan Cosmetic Industry Association, Limited also joined our efforts to revise the guidance as advisors, and we repeatedly discussed the content of the research.
Going forward, we are going to work together to provide more data and improve test conditions further and publish the obtained test results and knowledge as a database, in order to contribute to the cosmetics industry.
Background, results, and future outlook of the joint six-party research
In the cosmetics industry, the use of test methods utilizing alternatives to animal experiments is increasing at an accelerating pace and these methods are employed to evaluate quasi-drugs and cosmetics among others. Official evaluation methods set forth in such documents as the quasi-drug guidance issued by the Ministry of Health, Labour and Welfare are available for safety tests. For skin irritation tests, the "Guidance on Skin Irritation Evaluation Systems for Safety Evaluation of Quasi-drugs and Cosmetics (Pharmaceuticals and Crude Drug Council Notice 0422 No. 3)" (hereinafter referred to as "the Guidance") is available. Our six-company team has been building a database of results and knowledge of skin irritation tests, as a way to make the Guidance easier to use.
Assisted by the Japanese Society for Alternatives to Animal Experiments's 8th research grant concerning test method evaluation aimed to ensure the safety of cosmetics and other products, our team has been able to obtain more detailed irritation data about 24 ingredients, using our product – Human 3-Dimensional Cultured Tissue "LabCyte EPI-MODEL24."(Fig.1) Of these 24 ingredients, six were among the previously evaluated 16 ingredients and the other 18 ingredients were newly evaluated. The OECD Test Guideline 439*, which is used in the Guidance, is primarily intended to evaluate accidental skin exposure to an ingredient. Therefore, it assumes the evaluation of an undiluted ingredient and does not mention test conditions or any other items applicable when the ingredient is diluted. The Guidance does not permit ingredients to be diluted, either. Also, even if the concentration at which an ingredient becomes non-irritating when diluted is found, there is no clear criterion for setting a concentration that can be applied as skin irritation, which is considered as a problem as well.
Currently, we are conducting human patch tests, which the Guidance says should be performed after the procedure of the OECD Test Guideline 439, in cooperation with an external organization and validating data correlations. We intend to obtain data by combining results of tests using alternatives to animal experiments with results of human tests and, based on the obtained data, provide a set of data that can contribute to the revision of the Guidance.
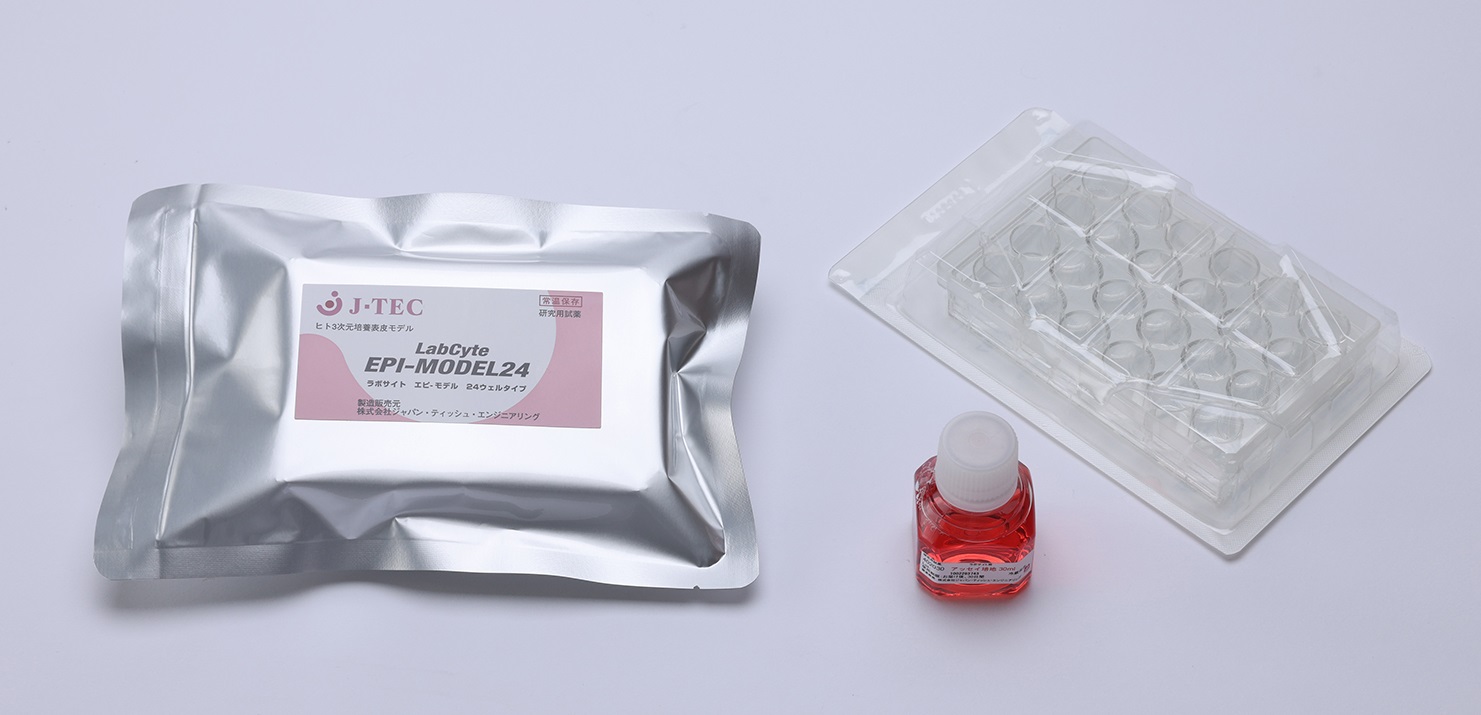

(Fig.1) Human 3-Dimensional Cultured Tissue "LabCyte EPI-MODEL24"
*OECD TG439: Organisation for Economic Co-operation and Development (OECD) adopted in vitro method for skin irritation “Test method using Reconstructed human Epidermis (RhE)” as OECD Test Guideline (TG) 439.
J-TEC's efforts toward alternatives to animal experiments
J-TEC manufactures and markets cultured human tissue for research use, which is developed by applying the high-level cell culture techniques that it has accumulated through the development of regenerative medical products such as autologous cultured epidermis and cartilage. The cultured human tissue for research use is a tissue model created by culturing human cells in vitro and reconstructing those cells. Since this model can reproduce a structure extremely similar to human tissue, it can be used in various types of experiments as a substitute for animals and simple cultured cells. The test methods using human 3-dimensional cultured tissue "LabCyte EPI-MODEL24" is listed in the following international test guidelines: OECD Test Guideline 431 (In Vitro Skin Corrosion), OECD Test Guideline 439 (In Vitro Skin Irritation), and OECD Test Guideline 442D (In Vitro Skin Sensitization).
We do the whole process internally, from the manufacture of cultured human tissue for research use to its marketing, thereby enabling quick delivery, thorough quality control, and stable product supply.
We will stay committed to promoting the spread of this product and contributing to the use of alternatives to animal experiments.
LabCyte: Human 3-Dimensional Cultured Tissue Model
https://www.jpte.co.jp/en/business/LabCyte/

