Use of Autologous Cultured Epidermis to treat epidermolysis bullosa (epidermolysis bullosa dystrophica and junctional epidermolysis bullosa) has been covered by insurance since July 1, 2019.
What is epidermolysis bullosa?
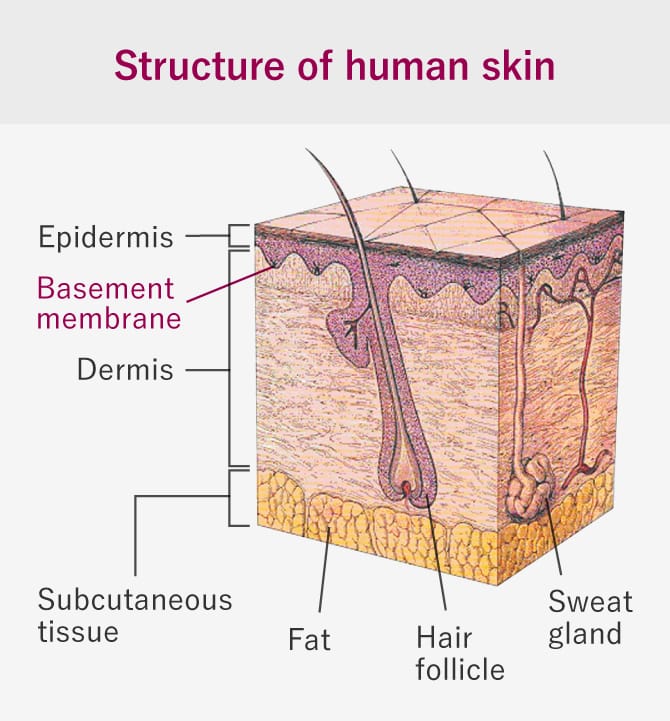
This is a rare and intractable disease of the skin in which the skin is structurally fragile from birth and slight mechanical stimulation causes erosion and ulcers. Skin tissue consists of 3 layers - epidermis, dermis, and subcutaneous tissue - with a basement membrane with adhesive functions between the epidermis and dermis (figure to right). In epidermolysis bullosa there is a gene mutation in the proteins that make up the basement membrane, which cases the skin to peel off easily in reaction to stimuli or friction so slight they would never cause damage under normal circumstances. In Japan there are thought to be 1,000 to 2,000 people with epidermolysis bullosa, including mild cases, and depending on the hereditary form and the position of the skin layer in which blisters form, these cases are classified into 3 main disease types: epidermolysis bullosa simplex, where blisters form in the epidermis, junctional EB, where blisters form between the epidermis and basement membrane, and dystrophic EB, where blisters form in the dermis.
Treatment methods
Symptoms of epidermolysis bullosa are varied, and apart from blisters and erosion, patients may also suffer from scarring, nutritional deficient, and anemia, etc. Treatment is tailored to the individual symptoms. Treatment for blisters consists of using a sterilized injection needle or scalpel to make a small hole in the blister membrane without breaking it, so as to release the fluid inside. The blister is then protected with ointment and gauze. The gauze must be changed daily. Other kinds of treatment include pain control, moisturization, oral care, eye care, nutritional supplementation, and surgery (for exacerbation of finger adhesion, esophageal stenosis, skin ulcers, etc.).
Methods using Autologous Cultured Epidermis (epidermal cell sheets) (Limited to dystrophic EB and junctional EB)
Part of the patient's own skin is cut away from an area without ulcers and cultured in a special facility to produce an epidermal cell sheet. The epidermal cell sheet is then grafted on to the area with erosion and ulcers after the affected area has been pretreated with a saline wash, etc. After the transplant, the wound will close in a few weeks or months. The epidermal cell sheet is a technology that was developed in the United States in 1975, and it has been covered by Japan's National Health Insurance for use in treating extensive burns since 2009 and for giant congenital nevocellular nevus since the end of 2016.

The use and commercialization of the medicinal products developed by Japan Tissue Engineering Co., Ltd. that are referred to on this website are approved only in Japan. A potential use and commercialization in other regions will be subject to the prior granting of a marketing authorization in the given territory and compliance with applicable laws.

